| |
Contents | EBJ Home | Single molecule measurements and biological motors |
Myosins are a class of molecular motors responsible for many motile processes in eukaryotic organisms. They are best known for their role in muscle contraction (Cooke, 1997) but there are at least 19 classes of myosins with a huge diversity of structures and mechanochemical properties (Hodge and Cope 2000; Berg et al., 2001; see also Myosin Home Page; Molecular Motors Page). Myosins produce force and displacement through their interaction with actin. Actin, a major constituent of the cytoskeleton, can form filaments which act as “tracks” for myosin-driven motility. The figure below shows the cyclical manner in which myosin is believed to interact with actin. The system of actin and myosin working together is referred to as actomyosin.
Despite many years of study, there are many issues which remain contentious, and others which are not fully explored, particularly with regard to the more recently discovered classes of myosins (Knight and Molloy, 2000). These include:
Power stroke - what is the displacement produced by a single interaction between myosin and actin? This is particularly contentious for muscle myosin, although current disagreement is “only” a factor of two - between ~5.5 and ~11 nm! (see for example Finer et al., 1994; Molloy et al., 1995; Kitamura et al., 1999).
Force - what is the force a myosin can produce? This has been a particularly difficult one to crack, due to series compliance problems. It is generally accepted to be a few piconewtons.
Directionality - most myosins seem to move towards the + or “barbed” end of the actin filament; myosin VI has recently been shown to move in the reverse direction (Wells et al., 1999; Homma et al., 2001).
Coupling - myosin is generally accepted to obtain convert chemical energy - in the form of ATP - into mechanical work. There has been much dispute over the amount of work obtained from a single ATP. Correlated single molecule measurements may be one way to resolve this issue (Ishijima et al., 1998).
Processivity - how many power strokes occur during one diffusional encounter between actin and myosin? Non-processive myosins work in large groups, detaching rapidly after completing their power strokes. Processive myosins (such as myosin V) remain attached for long periods, translocating for long distances along actin filaments (Mehta et al., 1999; Veigel et al., In Press).
Orientation dependence - does the power stroke or force produced by the myosin depend on the angle between the motor and the actin filament? This issue is significant for understanding motility assays, optical tweezers experiments and the action of non-muscle myosins.
Regulation - several modes of myosin regulation are known to exist, but in most cases it is not clear what the mechanism is. Investigating different regulatory states with single molecule experiments may help to elucidate this.
Cargo binding - all myosins need some way of transferring the work they do to their “cargo”, whether this be a thick filament in muscle or a vesicle in a cell. In many cases the mechanism and regulation of this cargo binding is not understood.
Cellular functions - in many cases the precise function of myosins is not understood. Even where a mutation in a myosin gene has a known phenotype, it may not be obvious how this relates to the myosin's function at the cellular level. In some cases the genes have been identified by other means and their function must be identified by experiment. This is often complicated by the fact that “knock-out” experiments may fail to reveal an obvious phenotype.
These are the issues which current single molecule experiments on myosins hope to resolve, in conjunction with biochemical, genetic and cell biological techniques.
 |
|
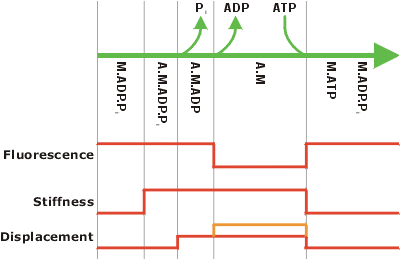
Schematic of the myosin mechanochemical cycle This figure shows the presumed correlation between the biochemical events of the myosin ATPase cycle, the stiffness changes, displacement steps and fluorescence from nucleotide analogues. The upper part shows the different steps in the biochemical pathway, with reaction co-ordinate proceeding from left to right (this is a subset of the full kinetic scheme and only shows the main pathway). M is myosin, A is actin and Pi is inorganic phosphate. The lower part shows the observable correlates of the pathway, as expected from the current model. The stiffness rises when myosin attaches to actin, and falls again when it detaches. An initial displacement might arise as phosphate is released, which occurs very rapidly after myosin binds to actin. A second displacement step (orange line) has been observed for some myosin I isoforms, and might also occur with smooth muscle myosin II (Veigel et al. 1999). This step may be coupled to the release of ADP. It is not clear whether skeletal muscle myosins lack this step or whether the rapidity of ADP release in these myosins means that the two steps have not yet been resolved. The fluorescence signal corresponds to the presence of nucleotide in the active site; it should therefore decrease on ADP release and be restored on ATP binding. However, some results (Ishijima et al. 1998) suggest that the fluorescence signal may drop before myosin binds to actin, which does not fit easily with the scheme shown here. |
| Contents | Next |